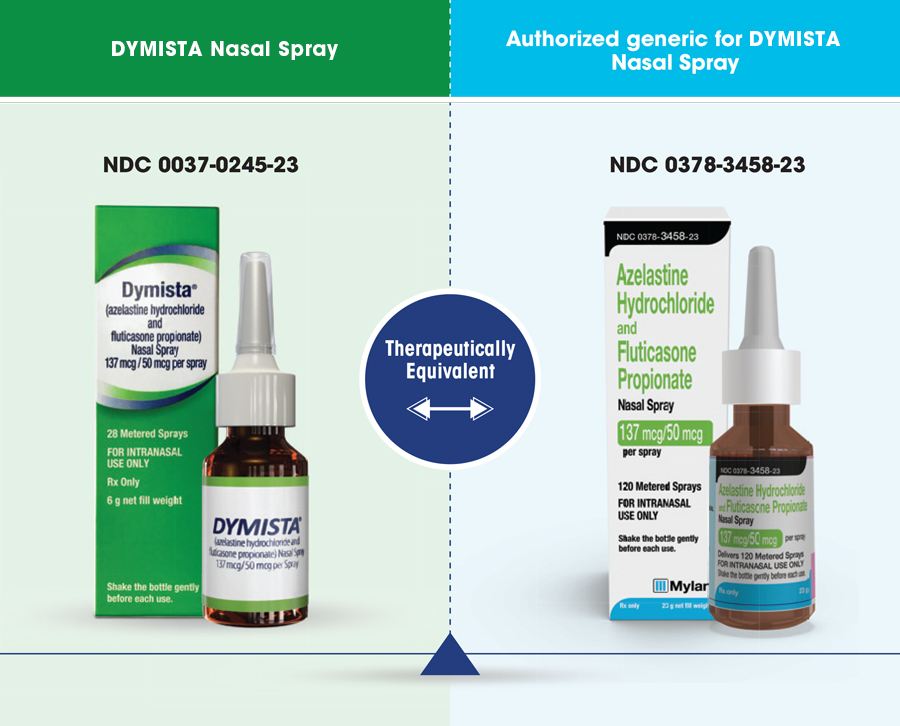
The Authorized Generic for DYMISTA® is the same formulation (combination azelastine hydrochloride and fluticasone propionate) nasal spray as the brand delivered in same vehicle.
Did You Know?
The term “authorized generic” drug is most commonly used to describe an approved brand name drug that is marketed without the brand name on its label. Other than the fact that it does not have the brand name on its label, it is the exact same drug product as the branded product. An authorized generic may be marketed by the brand name drug company, or another company with the brand company’s permission. In some cases, even though it is the same as the brand name product, a company may choose to sell the authorized generic at a lower cost than the brand name drug. Because an authorized generic drug is marketed under the brand name drug’s New Drug Application (NDA), it is not listed in FDA’s Approved Drug Products With Therapeutic Equivalence Evaluations (the Orange Book). While a separate NDA is not required for marketing an authorized generic, FDA requires that the NDA holder notify the FDA if it markets an authorized generic.1
Reference: 1. FDA List of Authorized Generic Drugs. Accessed June 2, 2021. https://www.fda.gov/drugs/abbreviated-new-drug-application-anda/fda-list-authorized-generic-drugs



