DYMISTA is contraindicated in patients with hypersensitivity to azelastine hydrochloride, fluticasone propionate, or to any other ingredients of DYMISTA. Reactions have included anaphylaxis.
Somnolence: Avoid engaging in hazardous occupations requiring complete mental alertness, such as driving or operating machinery, when taking DYMISTA.
Avoid concurrent use of alcohol or other central nervous system (CNS) depressants with DYMISTA because further decreased alertness and impairment of CNS performance may occur.
Epistaxis, nasal ulcerations, nasal septal perforation, impaired wound healing, Candida albicans infection: Monitor patients periodically for signs of adverse effects on the nasal mucosa. Avoid use in patients with recent nasal ulcers, nasal surgery, or nasal trauma.
Glaucoma or posterior subcapsular cataracts: Monitor patients closely with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
Potential worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infections, or ocular herpes simplex. More serious or even fatal course of chickenpox or measles in susceptible patients. Use caution in patients with the above because of the potential for worsening of these infections.
Hypercorticism and adrenal suppression with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue DYMISTA slowly.
Potential reduction in growth velocity in children. Monitor growth routinely in pediatric patients receiving DYMISTA.
Potent inhibitors of cytochrome P450 (CYP) 3A4: May increase blood levels of fluticasone propionate.
Ritonavir: Coadministration is not recommended.
Other potent CYP3A4 inhibitors, such as ketoconazole: Use caution with coadministration.
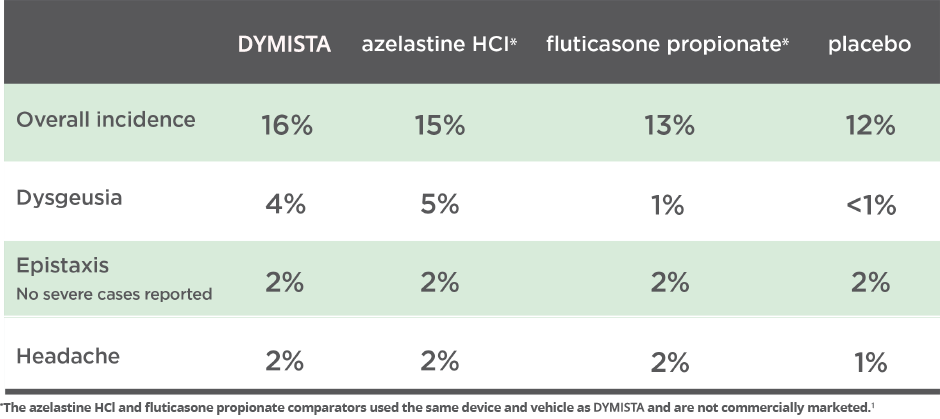
The most common adverse reactions (≥2% incidence) are dysgeusia, epistaxis, and headache.
INDICATIONS (The following information applies to DYMISTA and its Authorized Generic)
DYMISTA is indicated for the relief of symptoms of seasonal allergic rhinitis in adult and pediatric patients 6 years of age and older.
Please see
Full Prescribing Information.